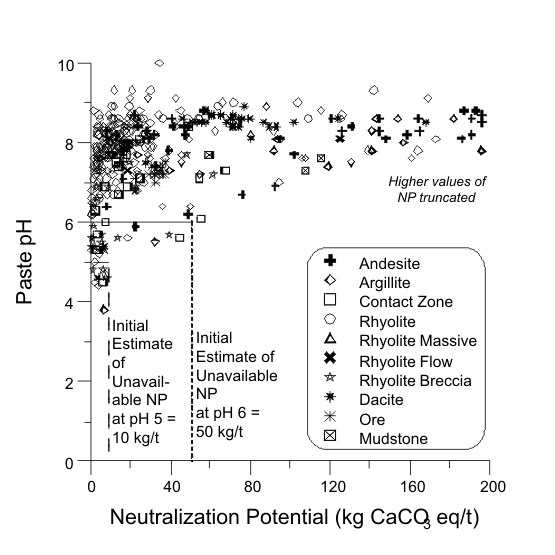
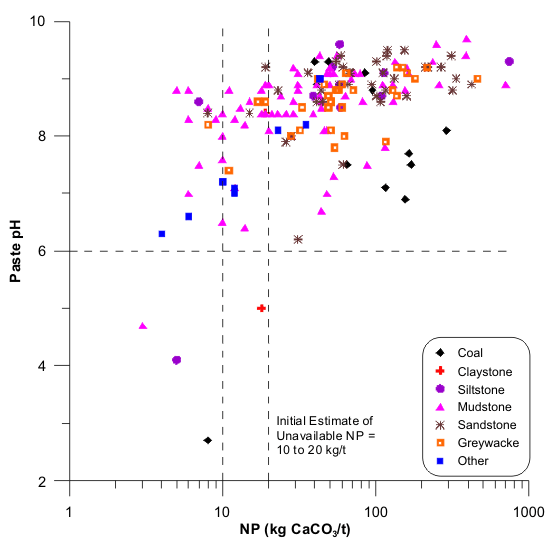
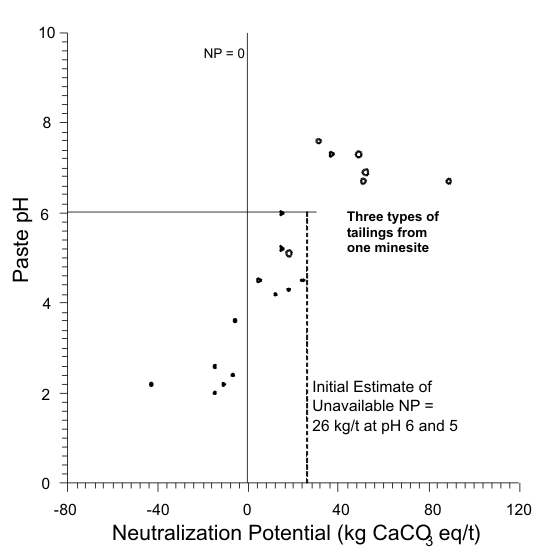
Figure 1. Estimation of Unavailable NP for Mine A, with Several Volcanic and Some Sedimentary Rock Units, and pH-Dependent UNPs.
MDAG.com Internet Case Study 30
Case Studies of Unavailable Neutralization Potential
in Acid-Base-Accounting Datasets
by K.A. Morin and N.M. Hutt
© 2008 Kevin A. Morin and Nora M. Hutt
www.mdag.com/case_studies/cs30.html
Click here for a PDF version of this MDAG.com Internet Case Study 30
Abstract
Unavailable Neutralization Potential (UNP) is defined as the portion of the measured Sobek
(EPA 600) NP that is not reactive or available under on-site field conditions. It can arise from
several factors, primarily:
- analytical-method artifacts,
- sample-specific conditions, like siderite,
- upscaling emergent effects, like the encapsulation of reactive NP rendering it unavailable at the
existing grain size.
This case study presents several examples from acid-base-accounting (ABA) datasets illustrating
the first two factors. The third factor is discussed in another case study, where 97% of measured NP
(or 97-130 kg/t) was unavailable.
The typical observed range of analytical-method and sample-specific UNP was 5 to 20 kg/t in ABA datasets, but many exceptions are known and some were shown. This approach works only if ABA samples were well oxidized and weathered before analysis, because fresh samples may not produce any net acidity. Also, UNP may not be apparent in ABA datasets due to factors like non-carbonate NP, mafic rock units, or drill-mud coatings.
The paste-pH criterion, on which UNP is estimated, can sometimes significantly affect the selected UNP value. Thus, the criterion should be identified.
In other cases, kinetic testing can show that the ABA-based UNP is incorrect. One example involved a tailings sample with a relatively high Sobek NP of 62 kg/t, but a paste pH of 4.4, suggesting all 62 kg/t were unavailable. However, subsequent humidity-cell testing showed pH recovered to near-neutral levels after the initial rinse at Week 0, and acidity fell substantially. This was attributed to thiosalt oxidation in the paste-pH solution after separation from the sample and before measurement. This is not an error, because it highlights issues affecting larger-scale minesite-drainage chemistry.
1. Introduction
Solid-phase Neutralization Potential (NP), also know as Acid Neutralising Capacity (ANC), is not a simple parameter. In fact, NP can be confusing and misleading. A large part of this stems from the fact that NP is not an element in the Periodic Table, but a concept created by humans and determined by arbitrary approaches. For example, NP can be estimated by titrations, but the types and endpoints of these titrations affect the results. Thus, there are many possible NP values for a particular sample.
One way to lessen this confusion is to recognize the “effective NP” as an intrinsic property of a sample. Effective NP can be defined as the capacity to neutralize acidity up to and above a pH of 6.0 under the site-specific environmental conditions, mineralogy, grain sizes, and rates of reaction (Morin and Hutt, 1997a, 2001, and in prep). Although rarely considered, effective NP can also be the capacity to neutralize alkalinity down to and below pH 9.0. These pH limits are arbitrary in that they simply coincide with many water-quality criteria, but still highlight the ambiguity that persists.
A key part of predicting pH in minesite-drainage chemistry through time is understanding
how to adjust any NP measurement to the Effective NP. A simple equation for this is:
Effective NP = Measured NP - Unavailable NP + Slow-Reacting NP (Eq. 1)
Unavailable NP (UNP) is defined as the portion of the measured analytical NP that is not
reactive or available under on-site field conditions (Morin and Hutt, 1997a, 2001, 2008, and in prep).
This has been defined for NP based on the Sobek et al. (1978) procedure, also known as the U.S.
EPA 600 procedure, and may not apply to other procedures. UNP can arise from several factors,
primarily:
- analytical-method artifacts,
- sample-specific conditions, like siderite,
- upscaling emergent effects, like the encapsulation of reactive NP rendering it unavailable at the
existing grain size.
A simple equation representing this is:
Total Unavailable NP (UNP) =
analytical-method UNP + sample-specific UNP + upscaling-effect UNP (Eq. 2)
2. Examples of Unavailable NP
This case study illustrates many examples of UNP derived from the first two factors of Equation 2, in acid-base-accounting (ABA) datasets. An example of the third factor, upscaling-effect UNP, under field conditions is presented in Morin and Hutt (2008), where 97% of measured NP (or 97-130 kg/t) was unavailable.
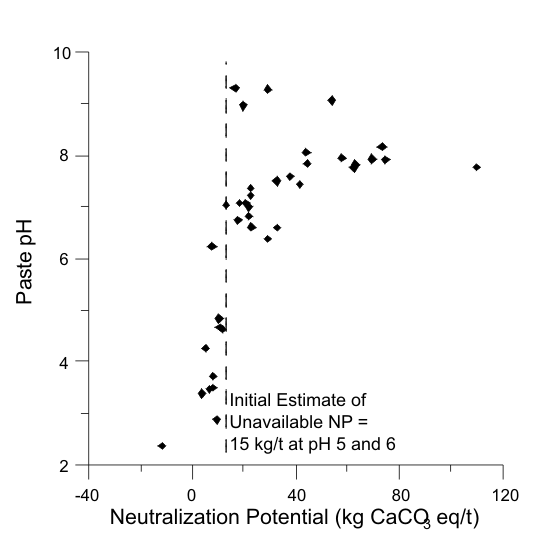
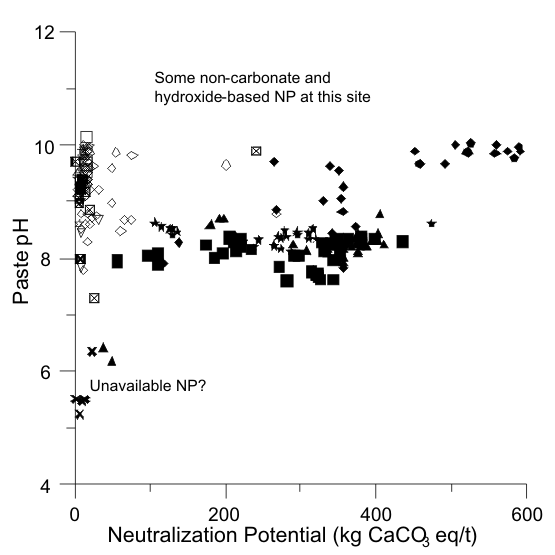
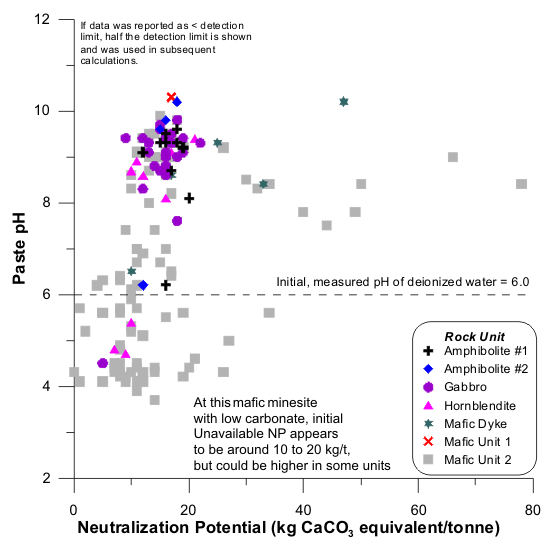
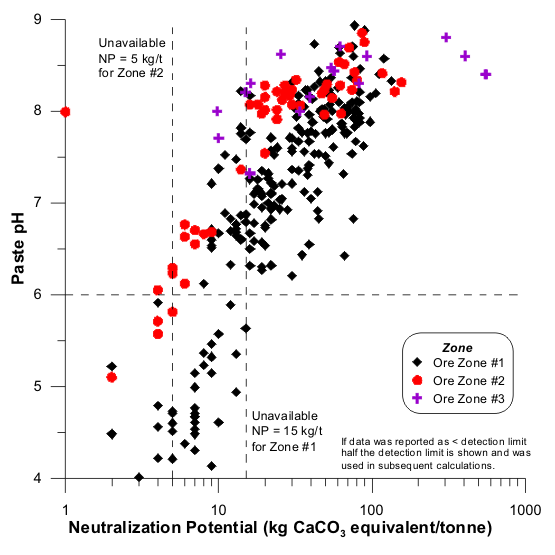
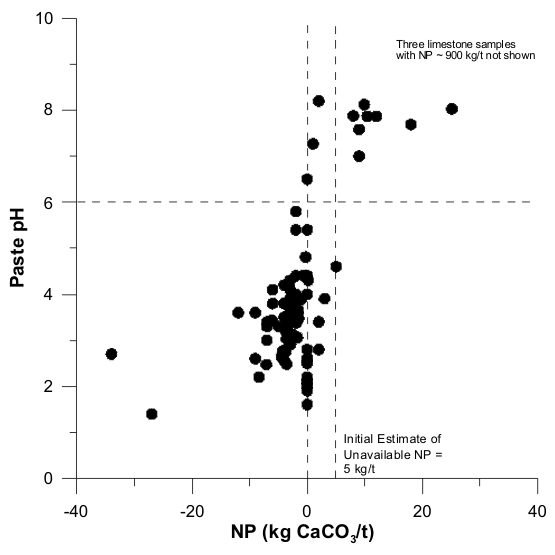
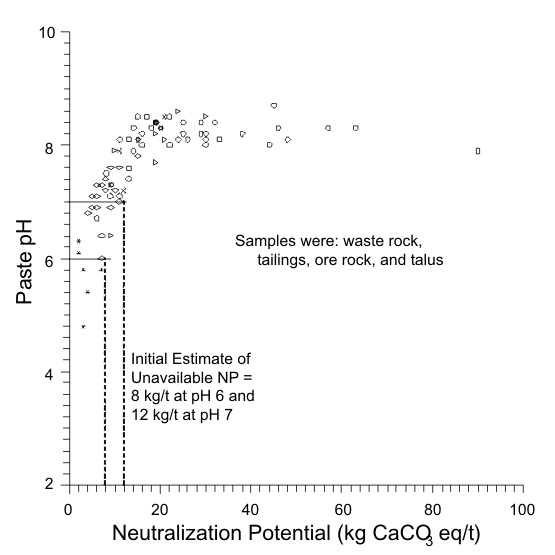
Figures 1 to 17 show scatterplots of measured Sobek NP against paste pH from ABA datasets. As NP decreased, some paste pH values decreased to acidic values. This provided an initial estimate of analytical-method and sample-specific UNP. The typical range of UNP is 5 to 20 kg/t, but many exceptions are known. This approach works only if samples were well oxidized and weathered before analysis, because fresh samples may not produce any net acidity.
The paste-pH criterion to define UNP in a particular dataset can vary, such as pH 5 or 6 or 7 (e.g., Figures 1, 2, and 13). For simplicity, a paste pH of 6.0 can be used if that is the applicable water-quality criterion or if Effective NP (Eq. 1) is based on it. Also, deionized water added to measure paste pH can be around pH 6, so any value below that would represent net acid generation. Still, local dilute rainwater could be around pH 5, so the paste-pH criterion of initial UNP should be identified for a particular site. Sometimes, this is not critical, because UNP can sometimes be independent of paste-pH criteria, at least between 5 and 6 (e.g., Figures 3, 5, and 6).
In some cases, an initial estimate of UNP is not apparent (e.g., Figures 4 and 7). This can be attributed to factors such as non-carbonate NP, mafic rock units, fresh unoxidized samples, or drill-mud coatings.
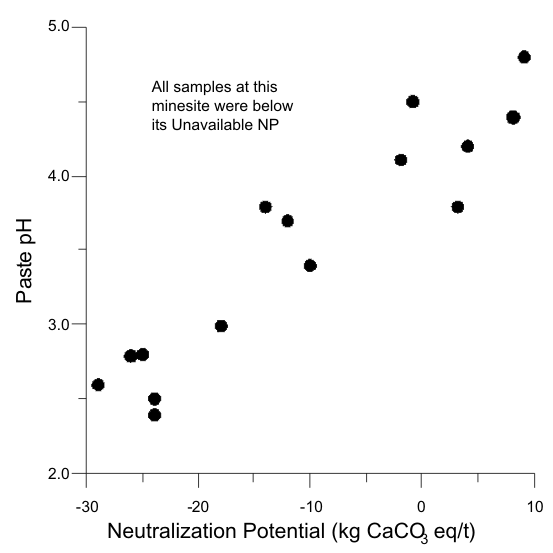
Sometimes all samples are below the UNP (Figure 17). In these cases, only a minimum bound for UNP, like >10 kg/t, can be determined.
In other cases, kinetic testing can show that the initial, ABA-based UNP is incorrect. This is obvious for cases where upscaling-effect UNP is greater than analytical-method UNP (Morin and Hutt, 2008). This is also the case where sample-specific UNP is greater. For example, a tailings sample had a relatively high Sobek NP of 62 kg/t, but a paste pH of 4.4 (Figure 18). The measured NP appeared completely unavailable. However, subsequent humidity-cell testing showed pH recovered to near-neutral levels after the initial rinse at Week 0, and acidity fell substantially. This tailings sample was known to contain elevated aqueous thiosalt concentrations. Thus, the oxidation of the paste-pH solution, after separation from the sample and before pH measurement, probably accounted for the misleading acidic pH. Nevertheless, this points to additional predictive complexities for overall drainage chemistry (Morin and Hutt, in prep).
3. References
Morin, K.A., and N.M. Hutt. In preparation. Minesite Drainage Chemistry.
Morin, K.A., and N.M. Hutt. 2008. Field Study of Unavailable Neutralization Potential in Acidic Rock. MDAG Internet Case Study #31, www.mdag.com/case_studies/cs31.html.
Morin, K.A., and N.M. Hutt. 2001. Environmental Geochemistry of Minesite Drainage: Practical Theory and Case Studies, Digital Edition. MDAG Publishing (www.mdag.com), Surrey, British Columbia. ISBN: 0-9682039-1-4.
Morin, K.A., and N.M. Hutt. 1997. Environmental Geochemistry of Minesite Drainage: Practical Theory and Case Studies. MDAG Publishing (www.mdag.com), Surrey, British Columbia. ISBN: 0-9682039-0-6.
Sobek, A.A., W.A. Schuller, J.R. Freeman, and R.M. Smith. 1978. Field and Laboratory Methods Applicable to Overburdens and Minesoils. Report EPA-600/2-78-054, U.S. National Technical Information Report PB-280 495. 403 p.









© 2008 Kevin A. Morin and Nora M. Hutt
For more case studies, see Environmental Geochemistry of Minesite
Drainage: Practical Theory and Case Studies.
Created by K.Morin